Importance and role of independent data monitoring committees (IDMCs) in oncology clinical trials | BMJ Open

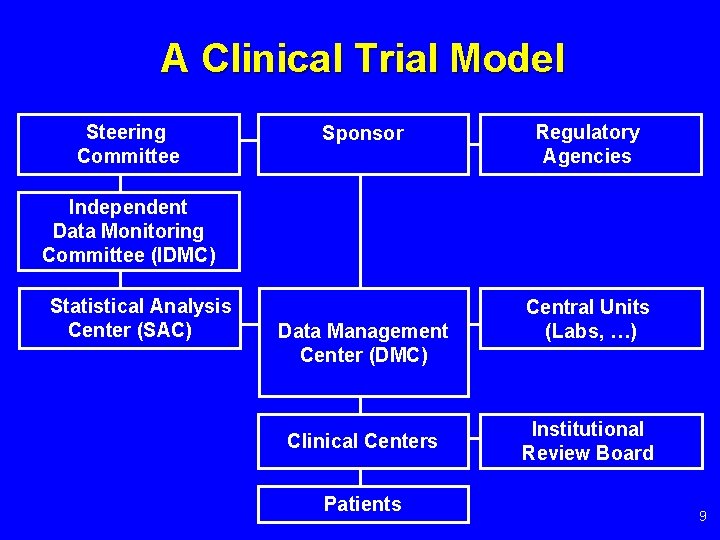
Independent Data Monitoring Committee (iDMC) and Role of A Biostatistician Irving K. Hwang, PhD ICG The rd Annual Meeting of DIA China Beijing, - ppt download

Formalising the induction of patient and public involvement contributors on trial oversight committees | Research Involvement and Engagement | Full Text
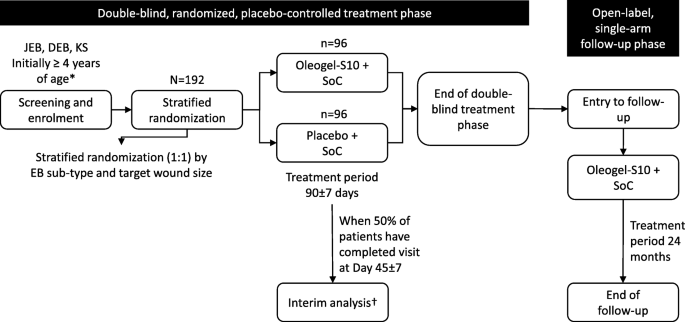
Oleogel-S10 Phase 3 study “EASE” for epidermolysis bullosa: study design and rationale | SpringerLink

Essential rules and requirements for global clinical trials in rare lung diseases: A sponsor׳s standpoint - ScienceDirect

New Trials If you are seeking a collaboration with the UCL CCTU we require you to apply: At least 3 months before the application deadline By using the. - ppt download

DATA MONITORING COMMITTEES IN CLINICAL TRIALS. Guidance for Research Ethics Committees - PDF Free Download

PPT - Independent Data Monitoring Committee (iDMC) and Role of A Biostatistician PowerPoint Presentation - ID:2295402

Importance and role of independent data monitoring committees (IDMCs) in oncology clinical trials | BMJ Open
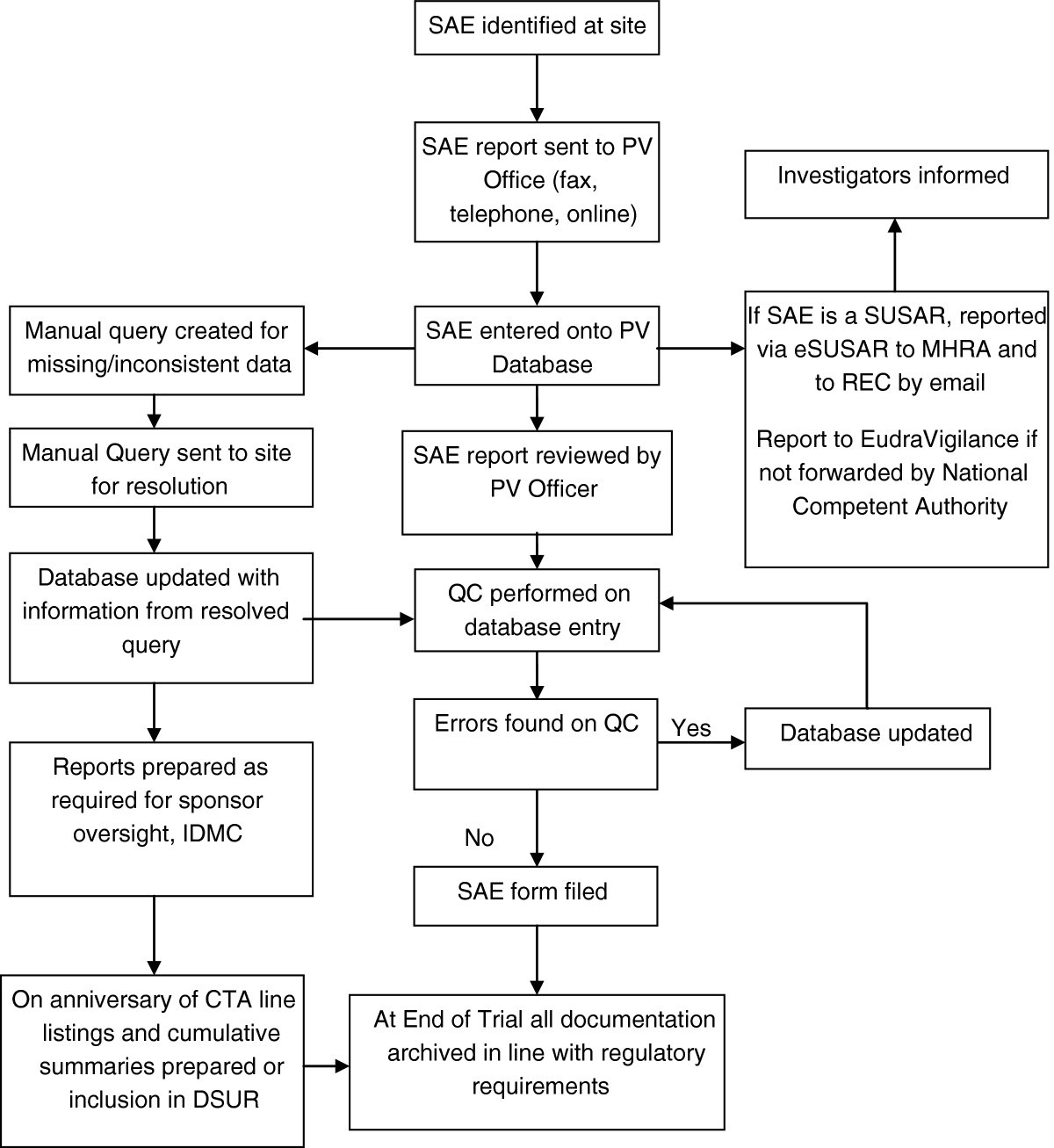
Implementing a centralised pharmacovigilance service in a non-commercial setting in the United Kingdom | Trials | Full Text




![Our Consortium [GANNET53] Our Consortium [GANNET53]](http://www.gannet53.eu/_media/raster_zusammenhang_eudario_fuer_webseite.png)