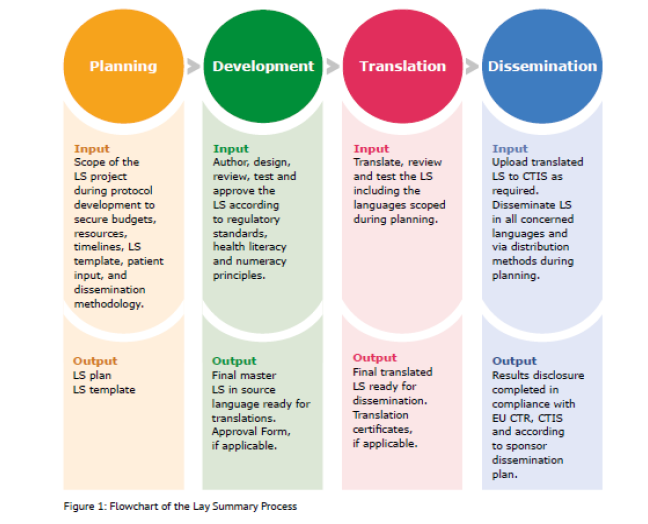
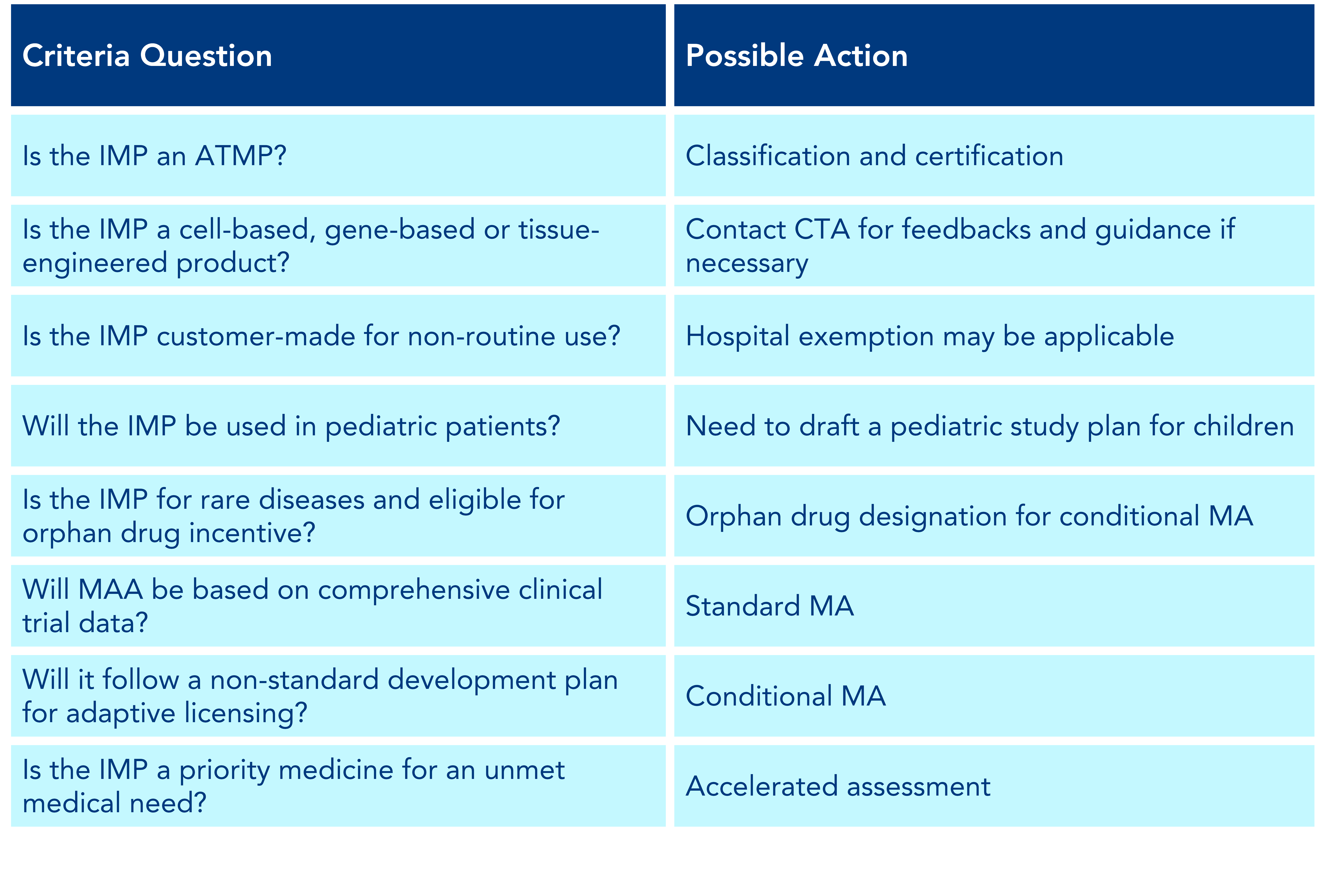
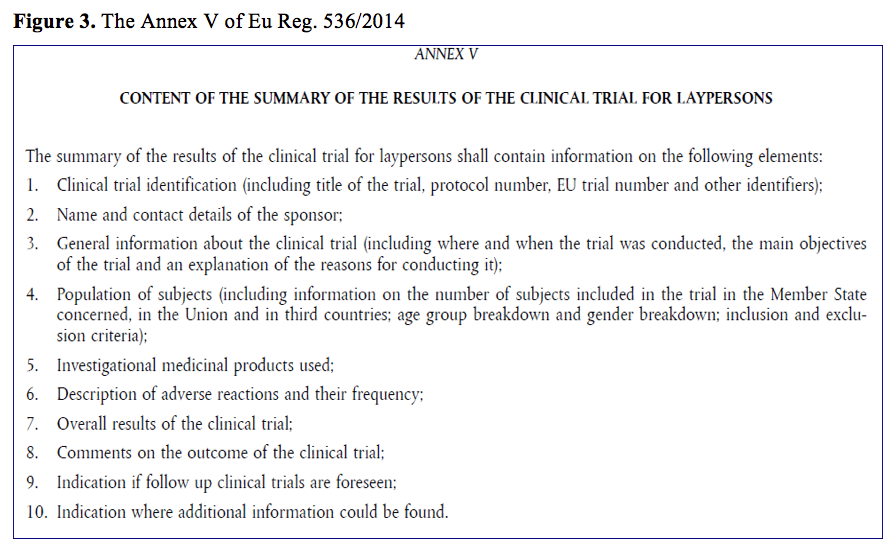
When innovation outpaces regulations: The legal challenges for direct‐to‐patient supply of investigational medicinal products - Malone - 2022 - British Journal of Clinical Pharmacology - Wiley Online Library
EudraLex The Rules Governing Medicinal Products in the European Union Volume 4 Good Manufacturing Practice Guidelines on Good M