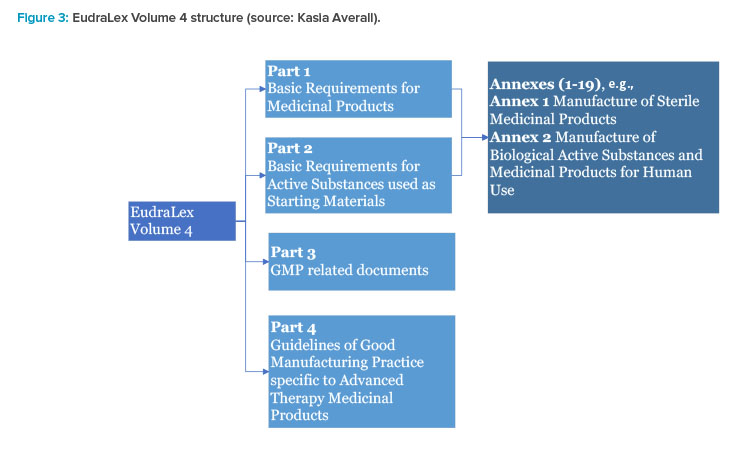
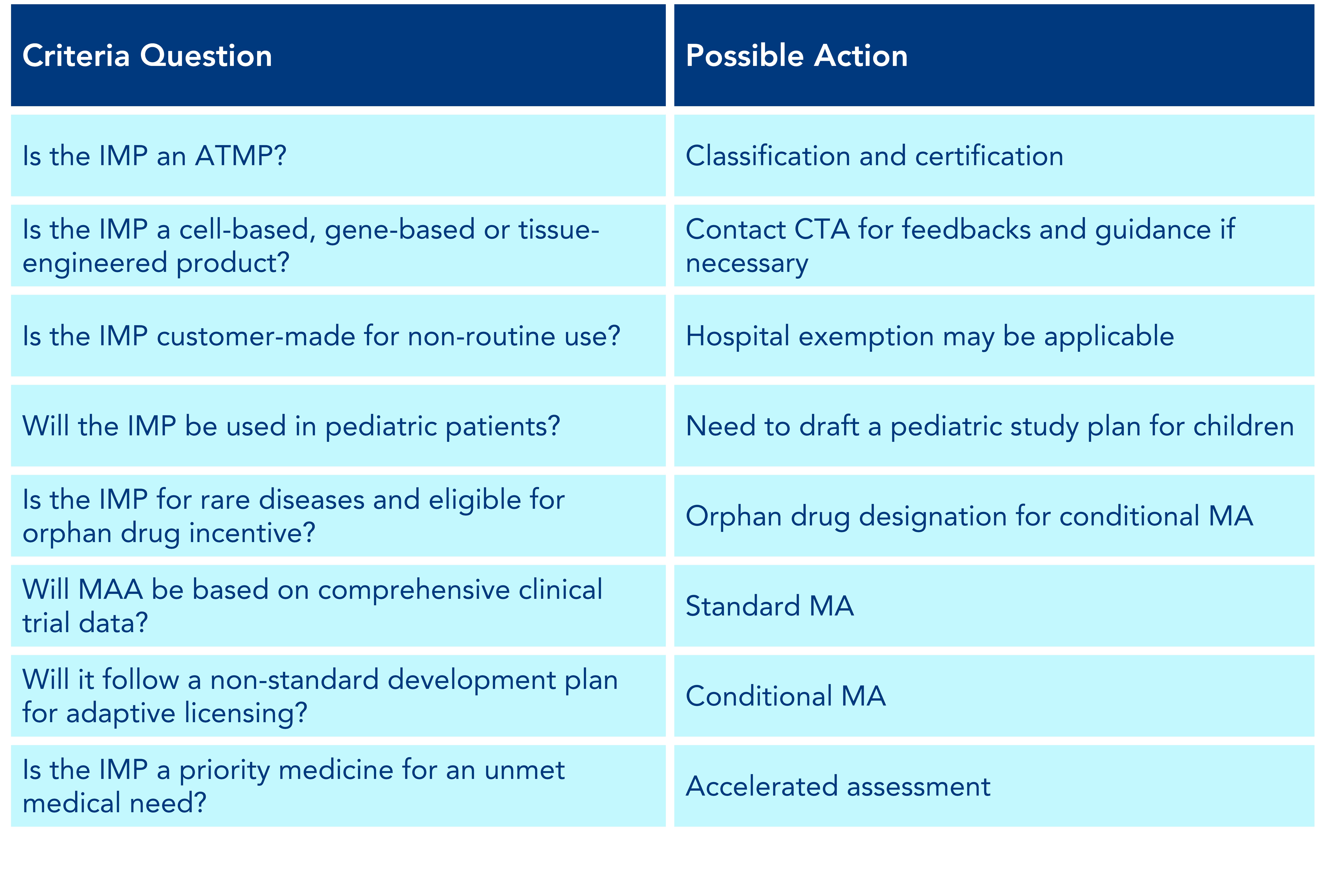
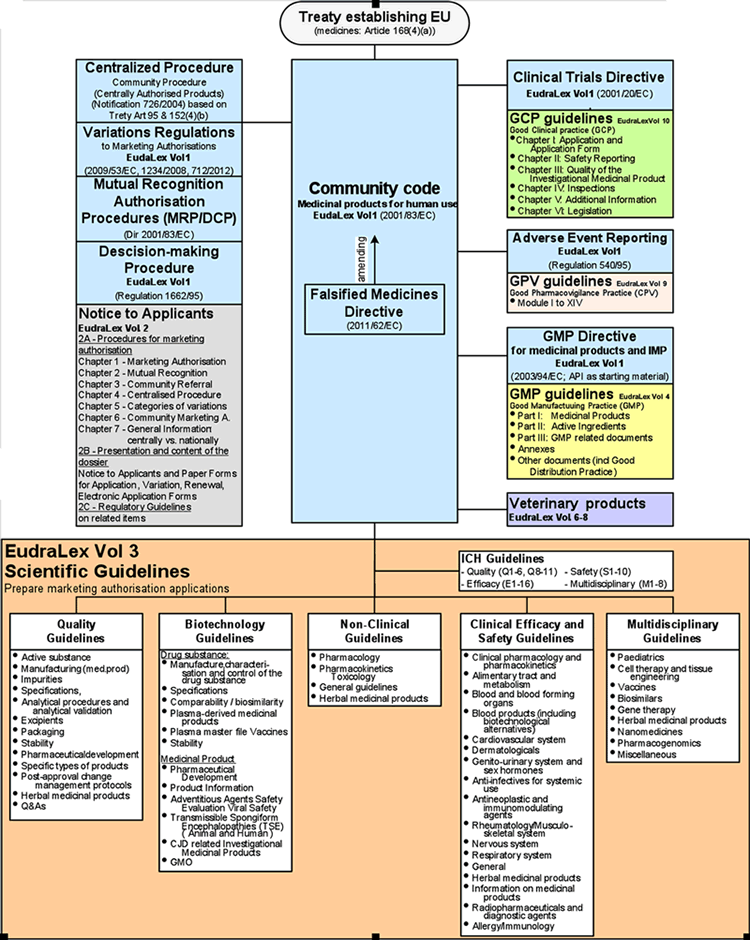
Principal Documents taken into account for the preparation of procedures for GCP inspections requested by the CHMP

GMP for medicinal products for human and veterinary use laid down in Commission Directives 91/356/EEC | M A N O X B L O G

EMA EudraLex - Volume 4 - GMP Guidelines - TELUGU GMP - Provides GMP Pharmaceutical Guidelines in Telugu.
EUROPEAN COMMISSION Brussels, 13 August 2014 Ares(2014)2674284 EudraLex The Rules Governing Medicinal Products in the European U

Frontiers | Advanced Therapy Medicinal Products' Translation in Europe: A Developers' Perspective | Medicine
EUROPEAN COMMISSION Brussels, 03 February 2010 EudraLex The Rules Governing Medicinal Products in the European Union Volume 4 Go