Advancements and Obstacles of CRISPR-Cas9 Technology in Translational Research: Molecular Therapy - Methods & Clinical Development
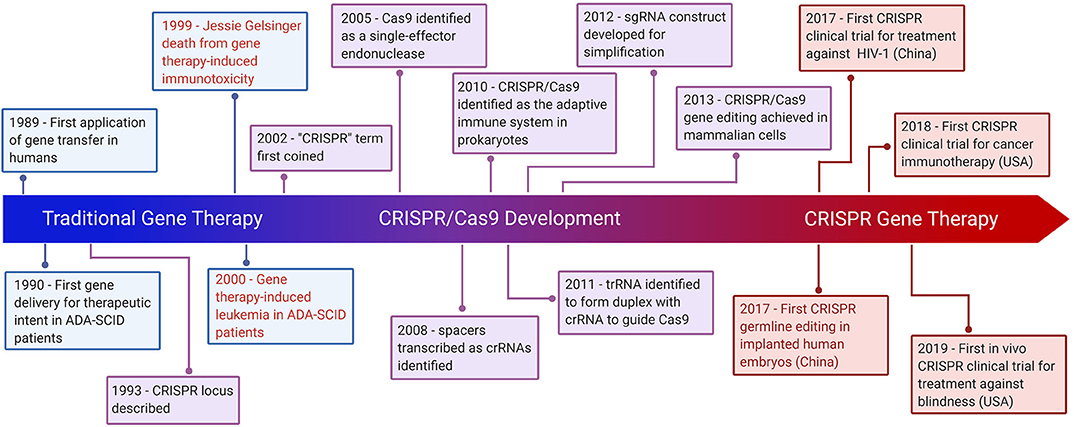
Frontiers | CRISPR Gene Therapy: Applications, Limitations, and Implications for the Future | Oncology
Clinical Data from Editas Medicine's Ongoing Phase 1/2 BRILLIANCE Clinical Trial of EDIT-101 for LCA10 to be Presented at the
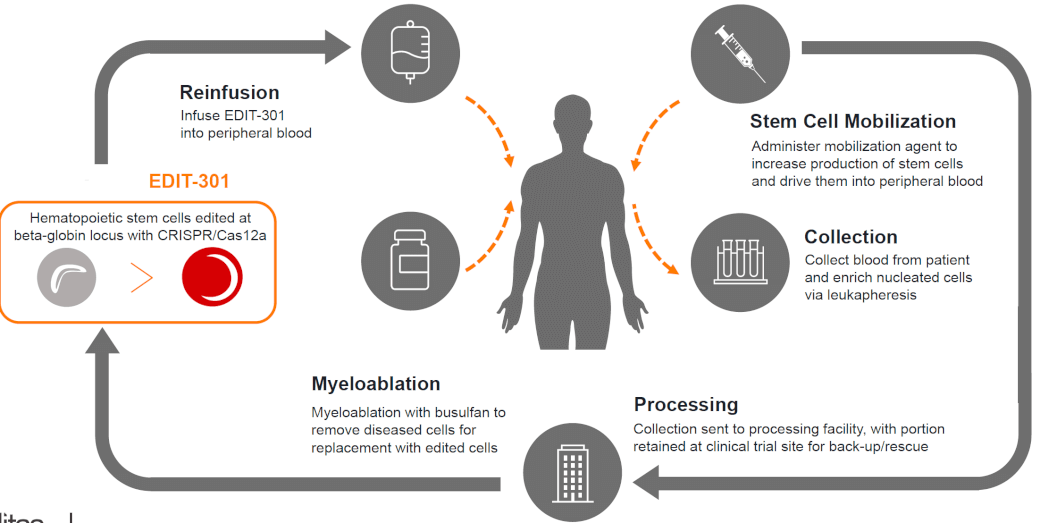
Amid The Turmoil Of 2020, This Company Was The First To Treat Patients With A Genome Editing Therapy