Completeness of Reporting of Patient-Relevant Clinical Trial Outcomes: Comparison of Unpublished Clinical Study Reports with Publicly Available Data | PLOS Medicine

Clinical comparison between trial participants and potentially eligible patients using electronic health record data: A generalizability assessment method - ScienceDirect

Quality Management of Electronic Systems in Clinical Trial Investigations: A Comparison of FDA and EMA Guidance - Life Science Training Institute

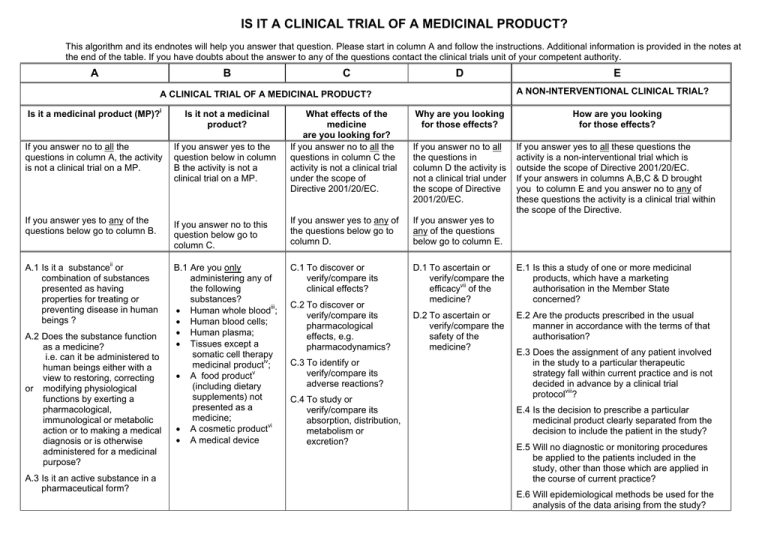
Comparison of clinical trial guidelines in USA, EU and India, Singapore. | Download Scientific Diagram
PLOS Medicine: Completeness of Reporting of Patient-Relevant Clinical Trial Outcomes: Comparison of Unpublished Clinical Study Reports with Publicly Available Data
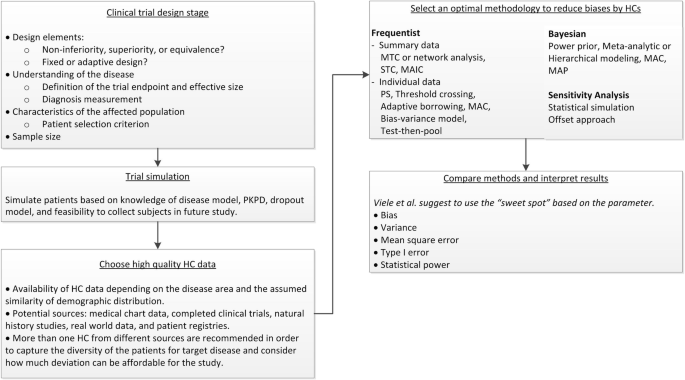
A roadmap to using historical controls in clinical trials – by Drug Information Association Adaptive Design Scientific Working Group (DIA-ADSWG) | Orphanet Journal of Rare Diseases | Full Text

60% cheaper clinical trials in Australia compared to US * CILIQUE - Clinical Outsourcing + Contract Management + CRO Vendor Selection & Management
Completeness of Reporting of Patient-Relevant Clinical Trial Outcomes: Comparison of Unpublished Clinical Study Reports with Publicly Available Data | PLOS Medicine
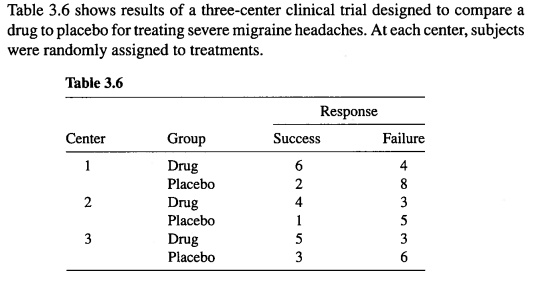
SOLVED:Table 3.6 shows results of three-center clinical trial designed to compare drug to placebo for treating severe migraine headaches. At each center; subjects were randomly assigned tO treatments. Table 3.6 Response Success