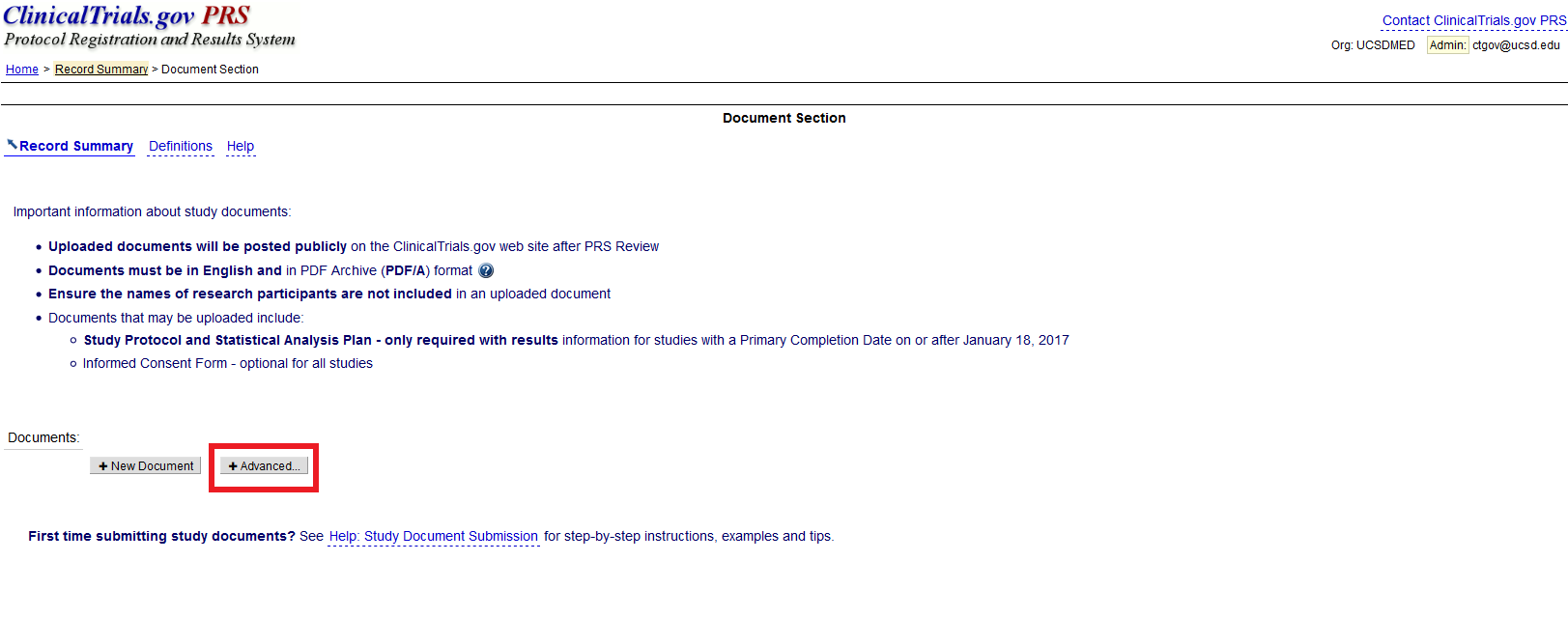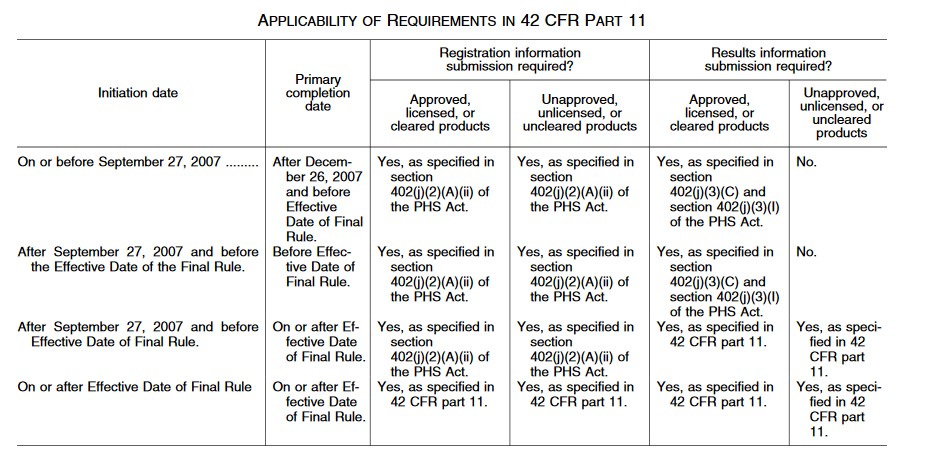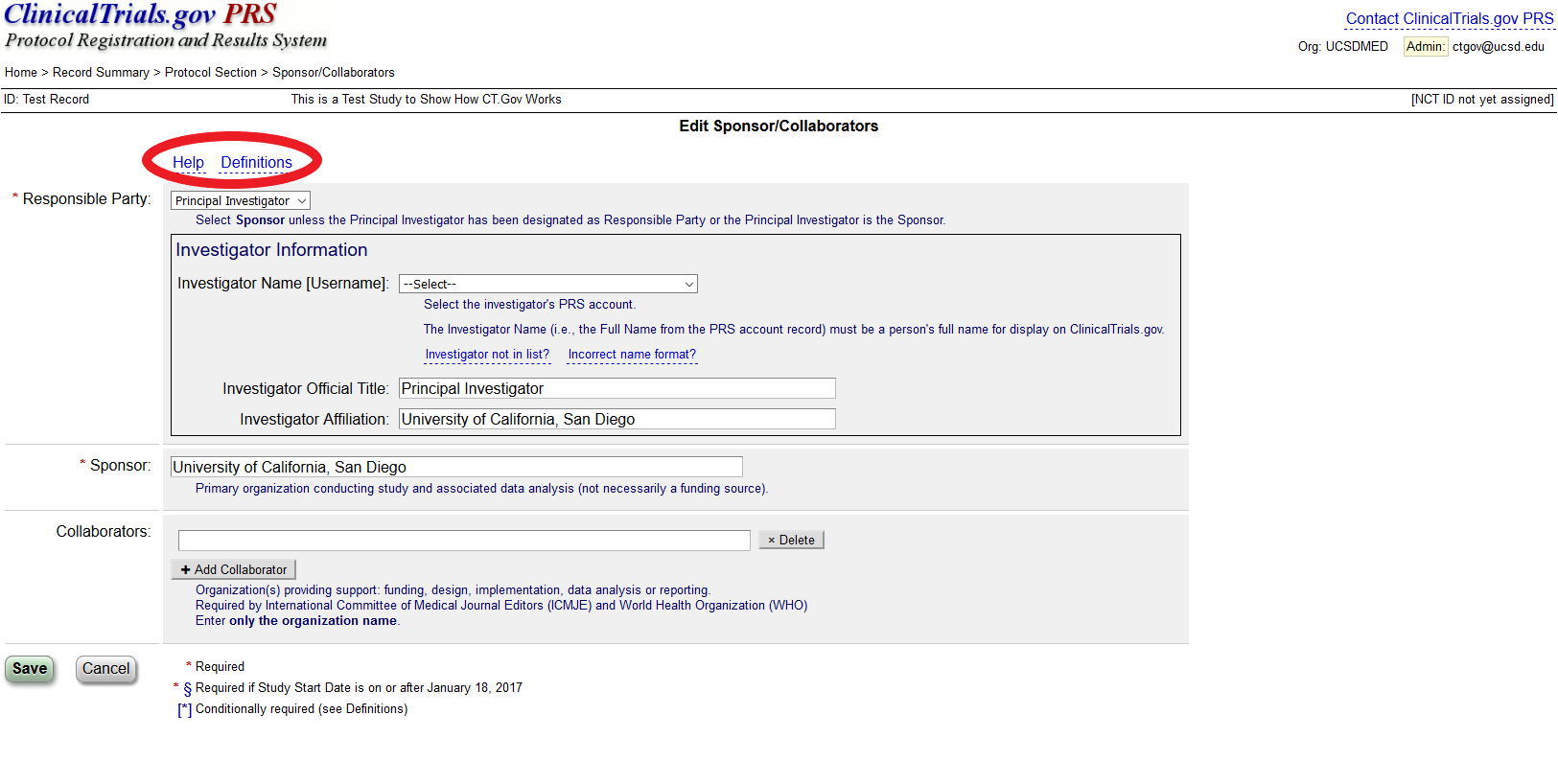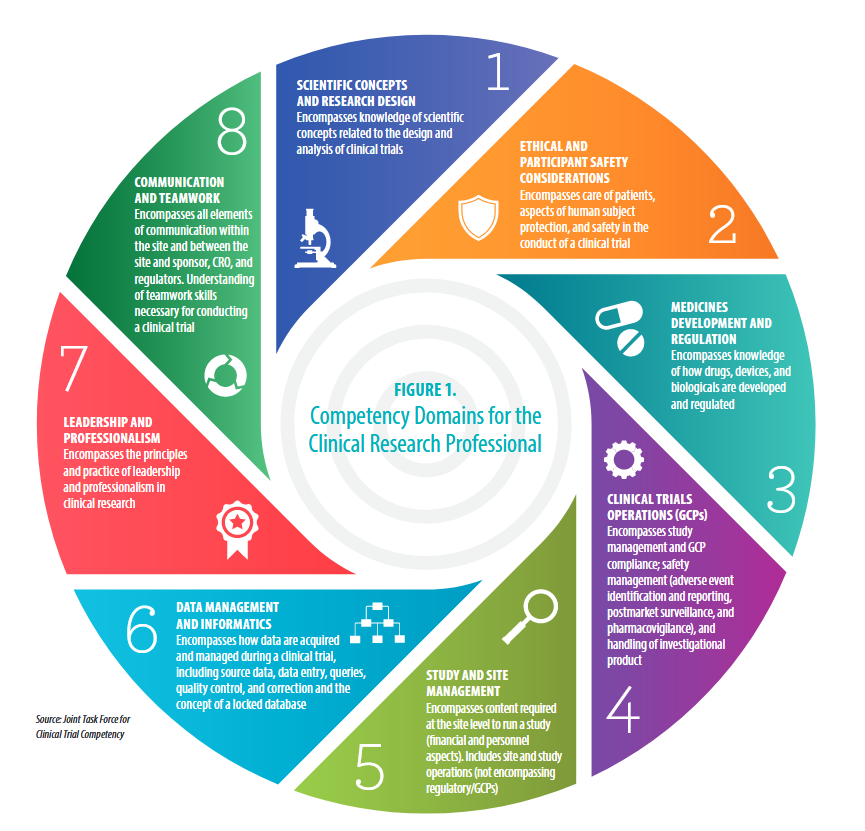
ClinicalTrials.gov Registration" | Center for Clinical and Translational Science | University of Illinois Chicago

Navigating the Expanding Regulations of ClinicalTrials.gov Registration and Results Reporting - Advarra

PPT - Everything You Need to Know About Clinical Trials Registration and Results Reporting Requirements PowerPoint Presentation - ID:4567826






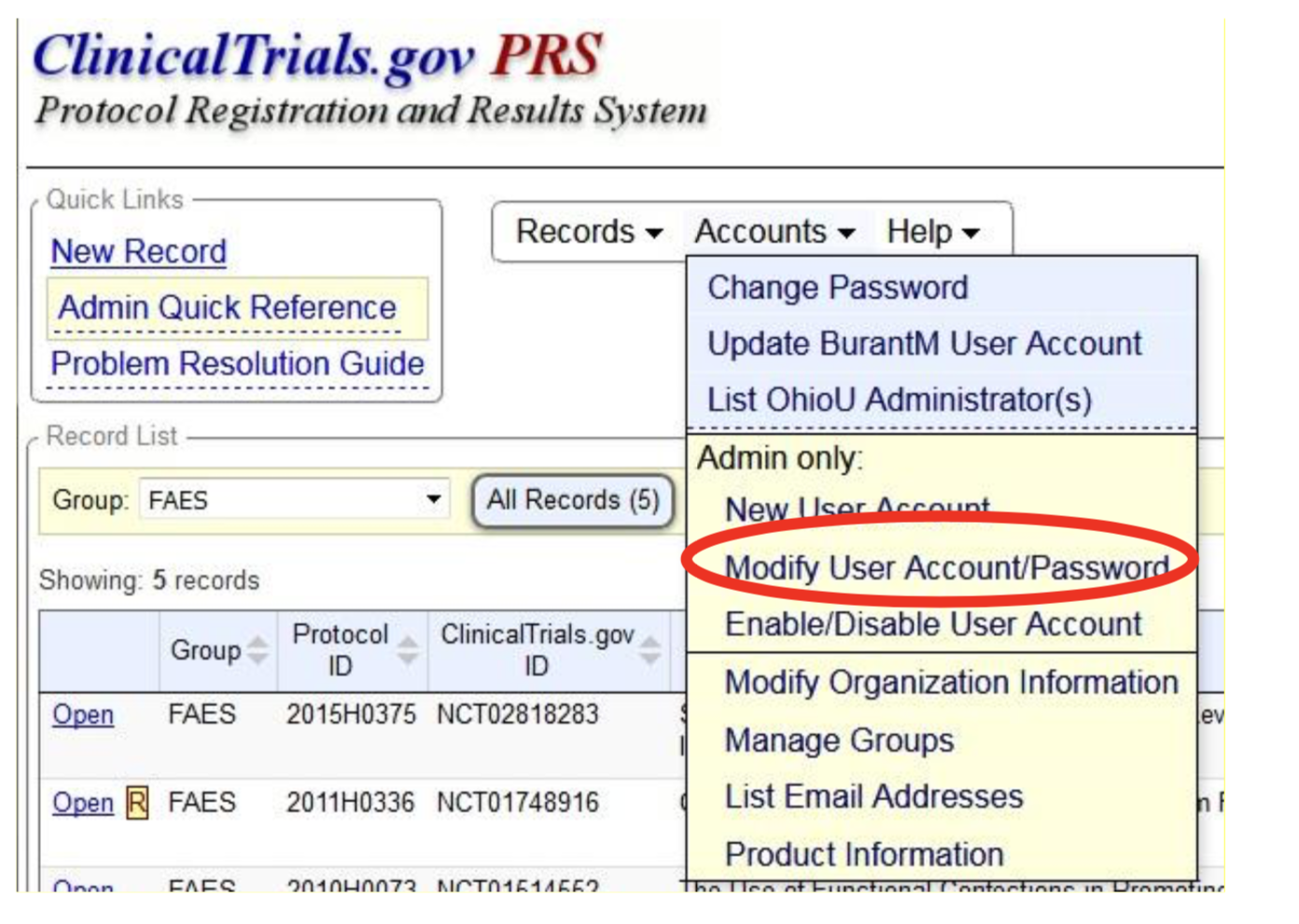
![PDF] Registering a clinical trial in ClinicalTrials.gov. | Semantic Scholar PDF] Registering a clinical trial in ClinicalTrials.gov. | Semantic Scholar](https://d3i71xaburhd42.cloudfront.net/7dd504f5349494c9b399e708b83f6effaae7ee9d/3-Table2-1.png)