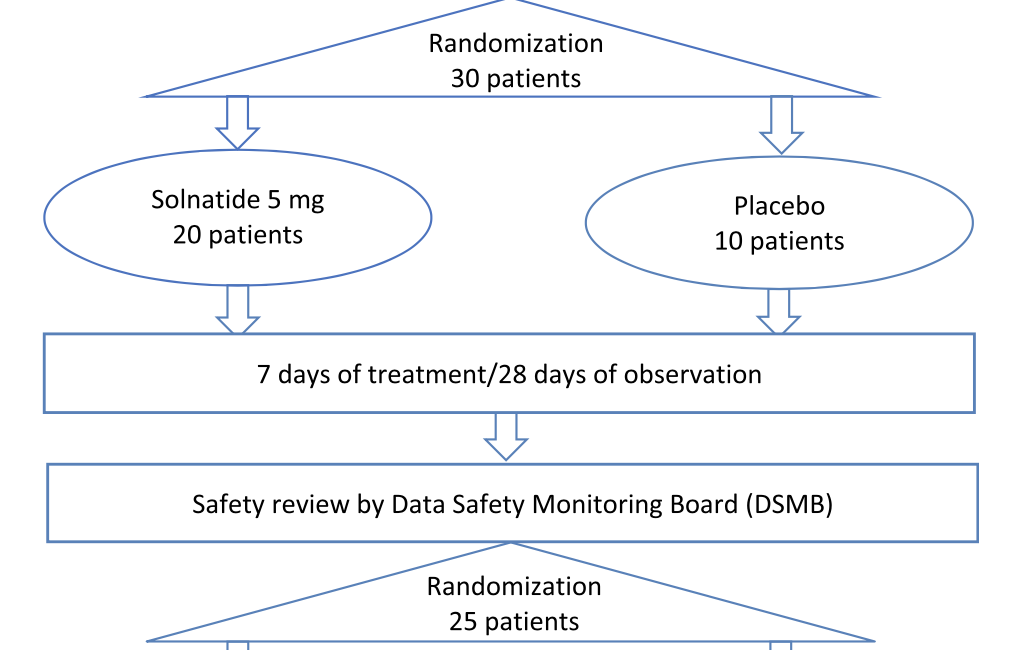
Clinical Trial Documentatino | Clinical trials study, Clinical trials, Contract research organization
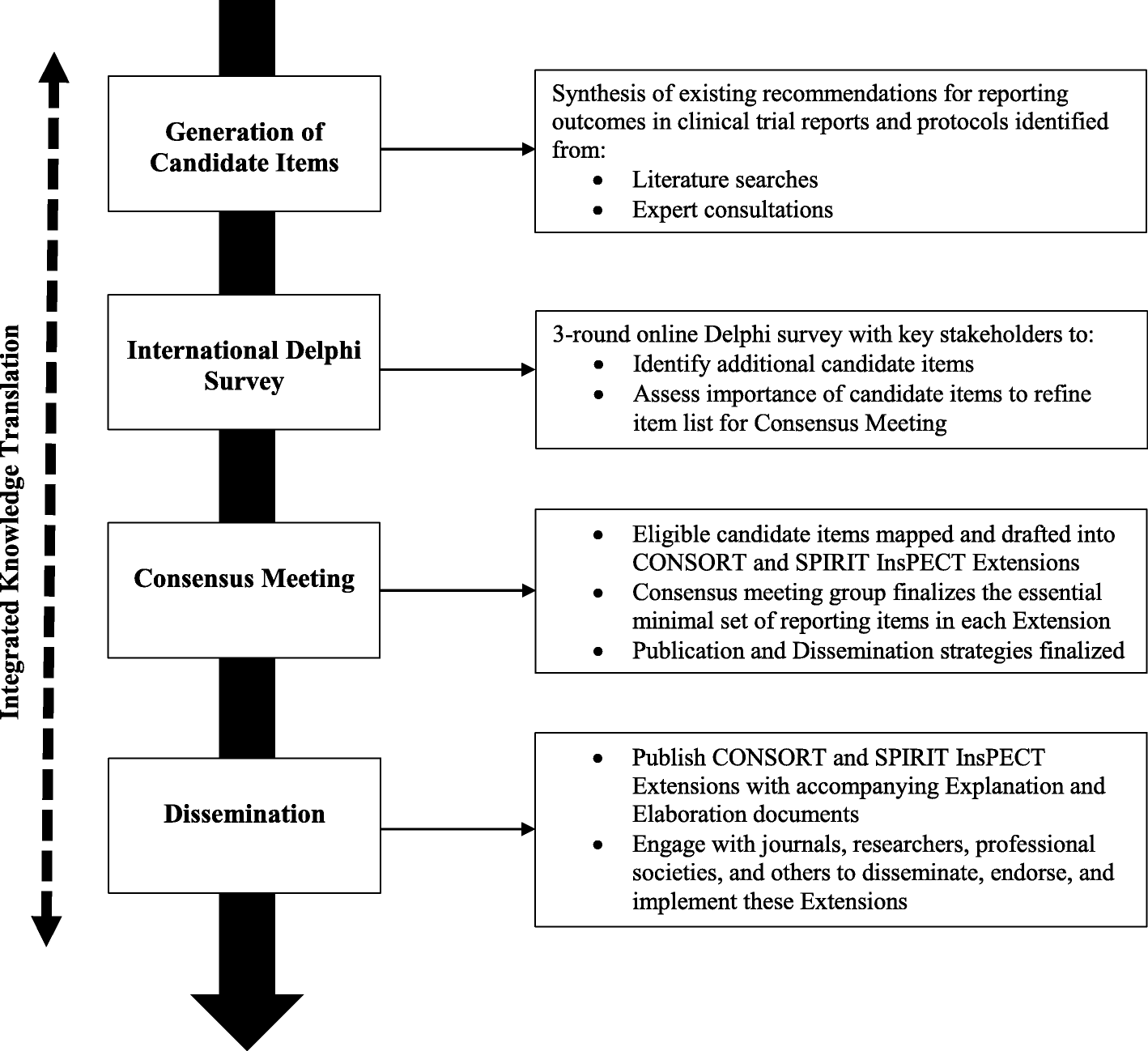
Improving outcome reporting in clinical trial reports and protocols: study protocol for the Instrument for reporting Planned Endpoints in Clinical Trials (InsPECT) | Trials | Full Text
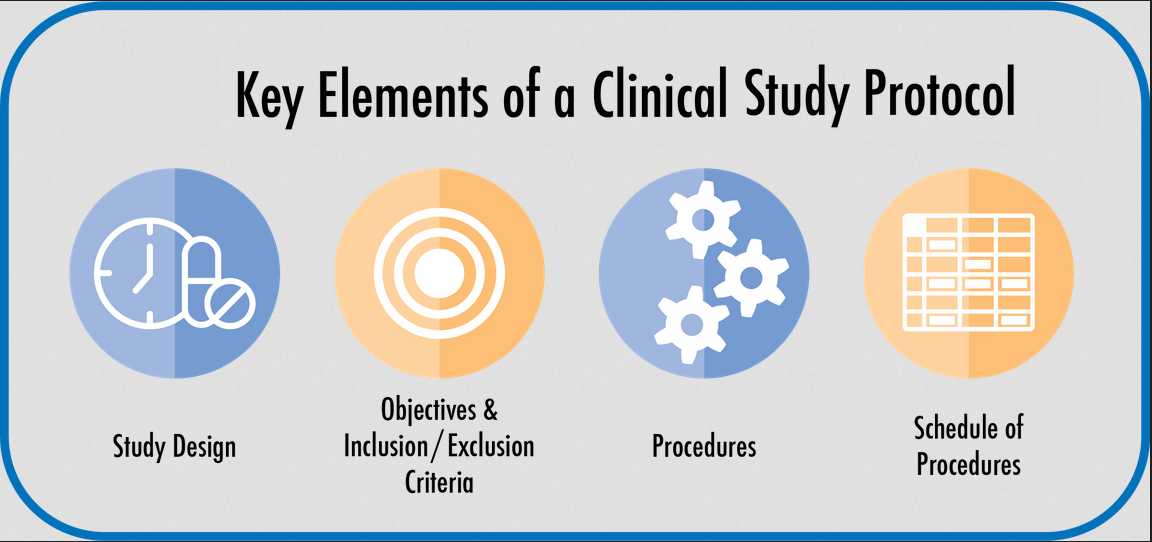
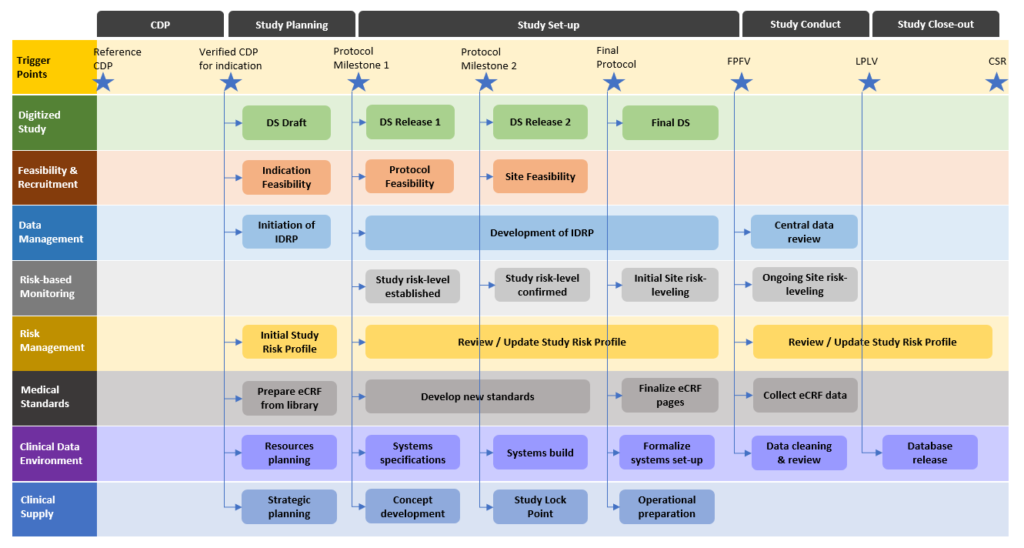
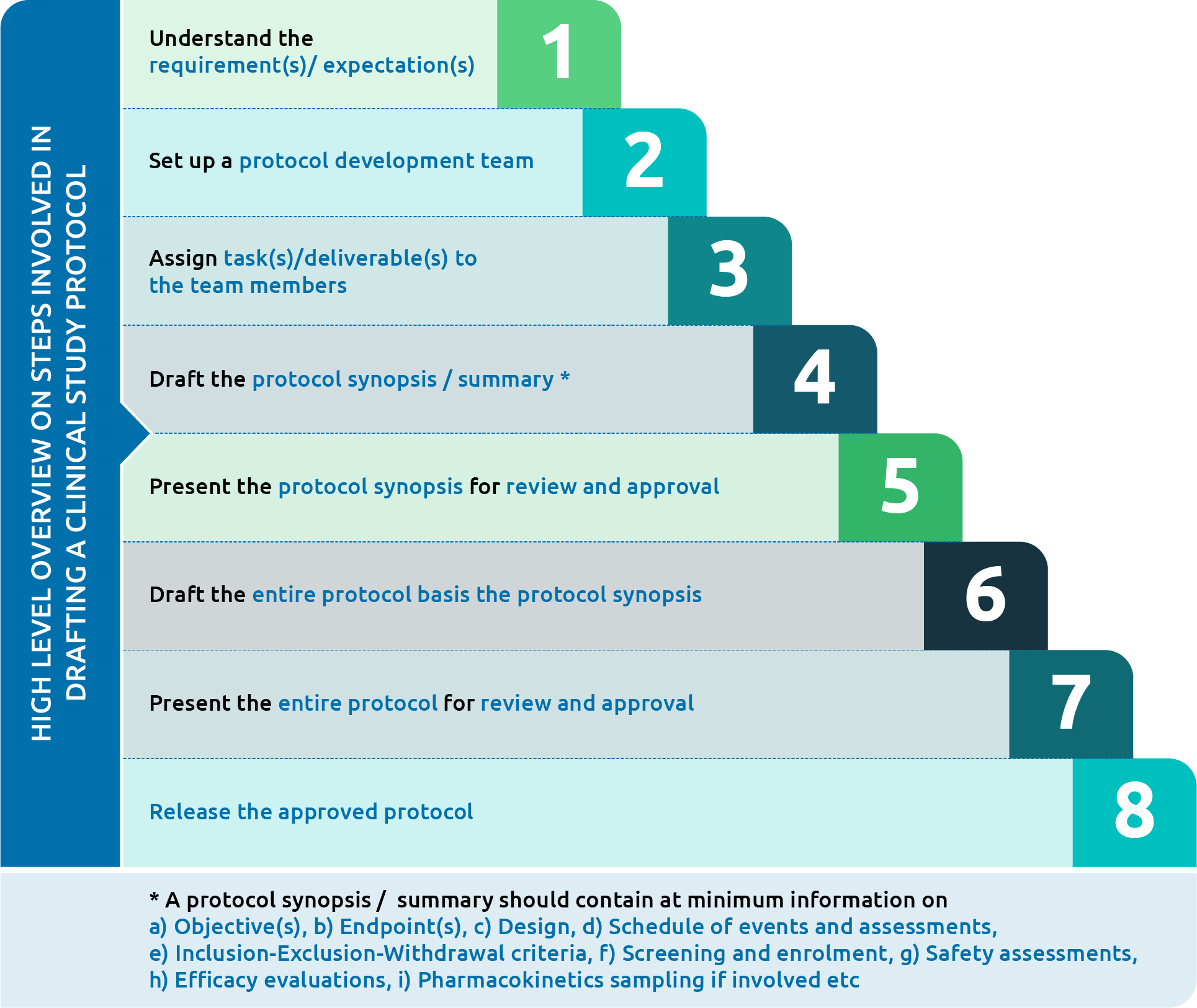
Clinical Trial Documentation – Clinical Study Protocol | AuroBlog | Clinical Research Blog | Aurous HealthCare CRO, India
An interactive retrieval system for clinical trial studies with context-dependent protocol elements | PLOS ONE
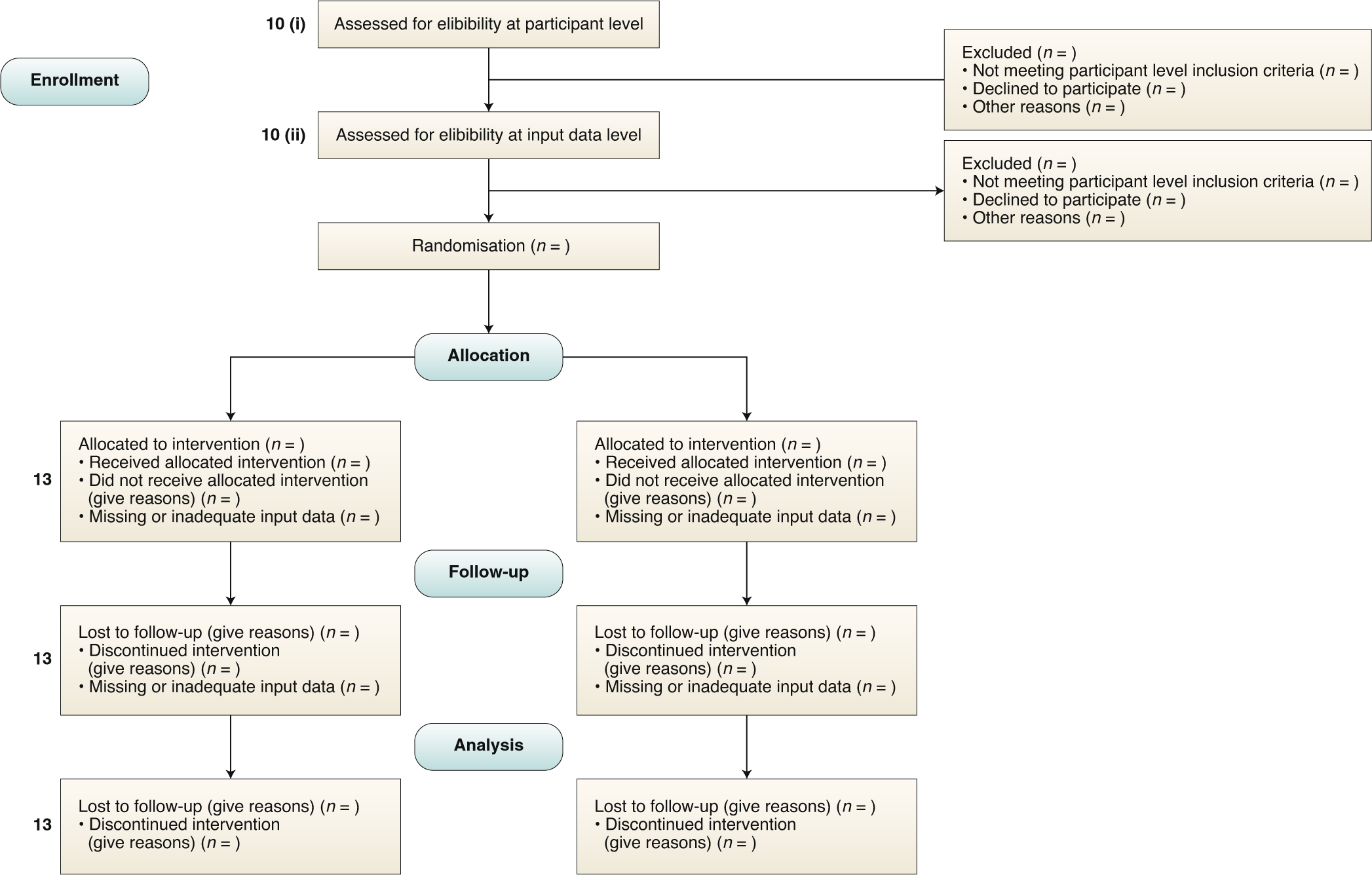
Guidelines for clinical trial protocols for interventions involving artificial intelligence: the SPIRIT-AI extension | Nature Medicine

Guidelines for clinical trial protocols for interventions involving artificial intelligence: the SPIRIT-AI extension - The Lancet Digital Health