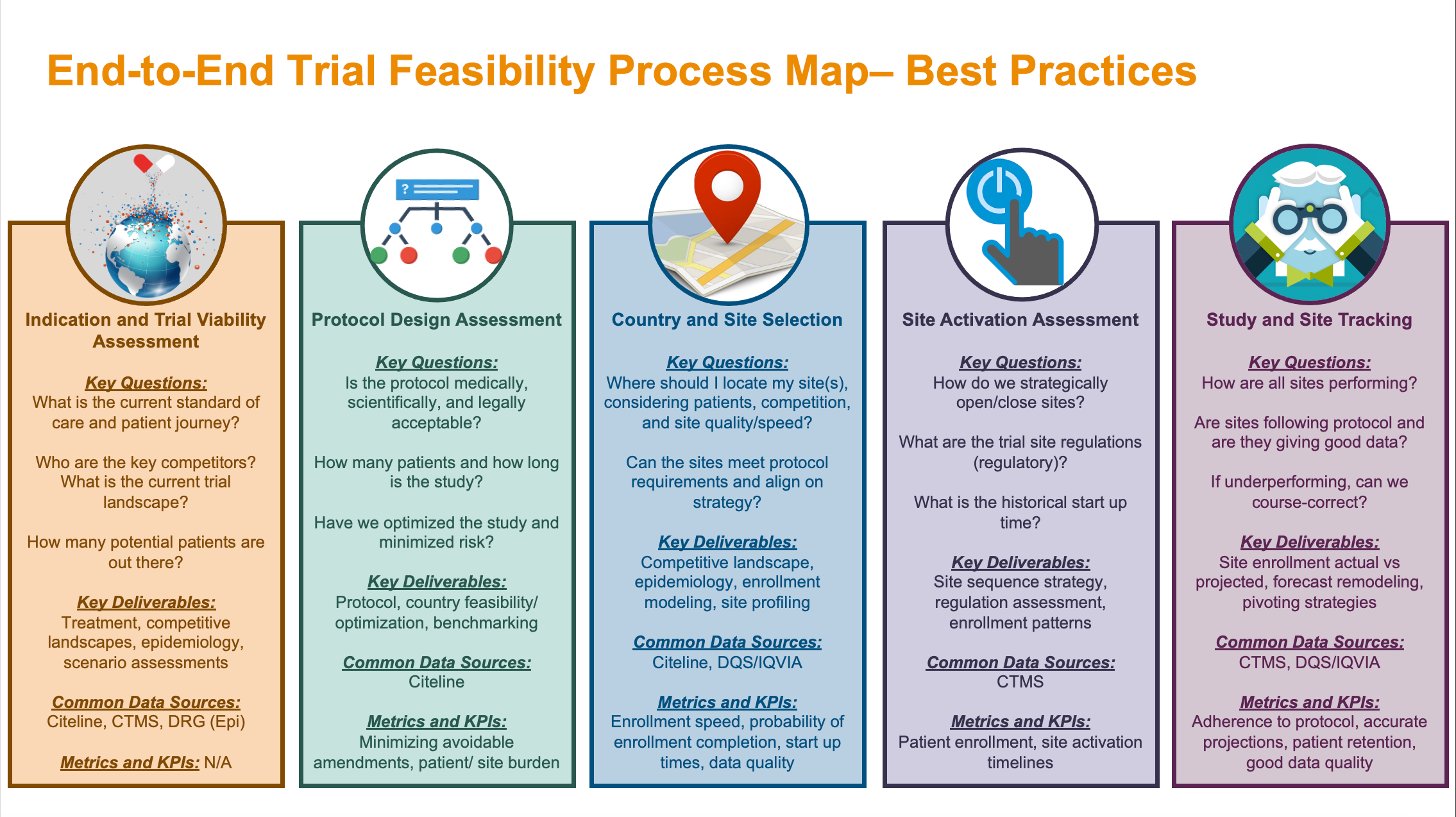
Clinical Study Protocol A Multicenter, Randomized, Double-Blind, Double-Dummy, Placebo- Controlled, Parallel-Group Study Compari

SOPs for GCP-Compliant Clinical Trials: A Customizable Manual for Sponsors of Medical Device Trials : MS Word Template | CenterWatch
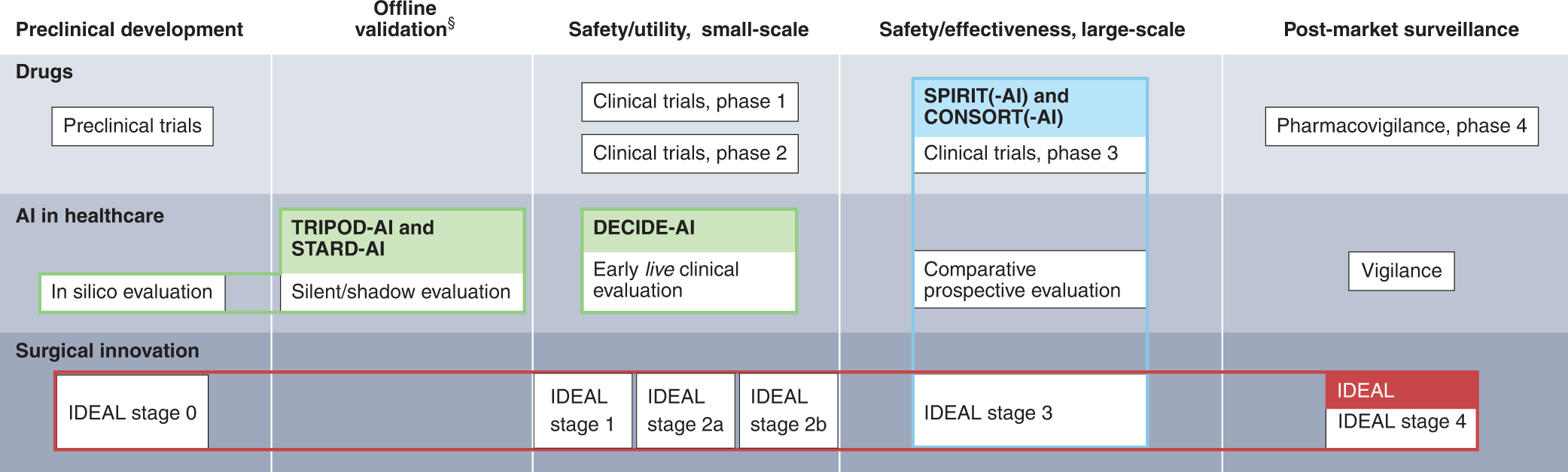
Reporting guideline for the early-stage clinical evaluation of decision support systems driven by artificial intelligence: DECIDE-AI | Nature Medicine
Guidance for Deploying Medical Image-based Software – including Algorithms – in the Clinical Setting

Guidelines for clinical trial protocols for interventions involving artificial intelligence: the SPIRIT-AI extension - The Lancet Digital Health