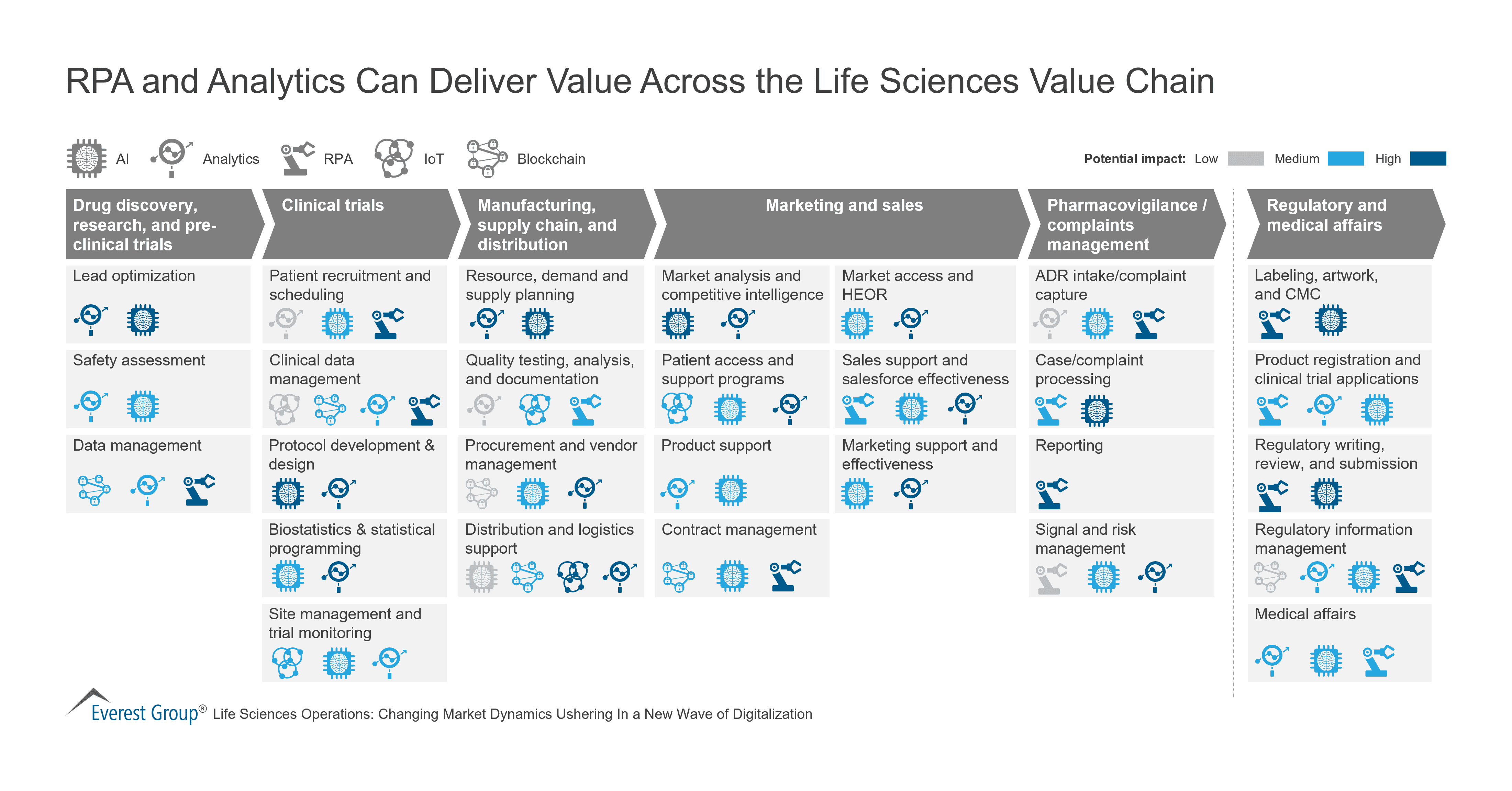
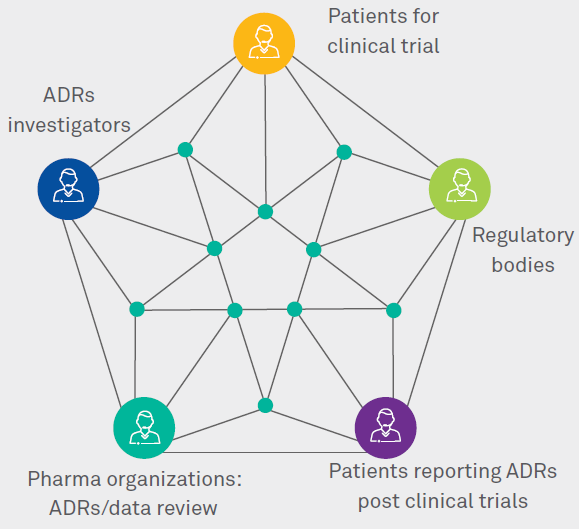
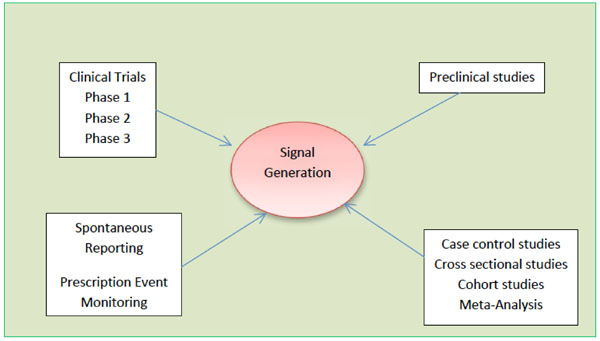
Variation in adverse drug reactions listed in product information for antidepressants and anticonvulsants, between the USA and Europe: a comparison review of paired regulatory documents | BMJ Open

Drug lit ADRs - Drug lit ADR lecture notes - Drug Literature Evaluation Adverse Drug Reaction + Drug - StuDocu

Adverse Drug Reactions to Guideline-Recommended Heart Failure Drugs in Women: A Systematic Review of the Literature - ScienceDirect
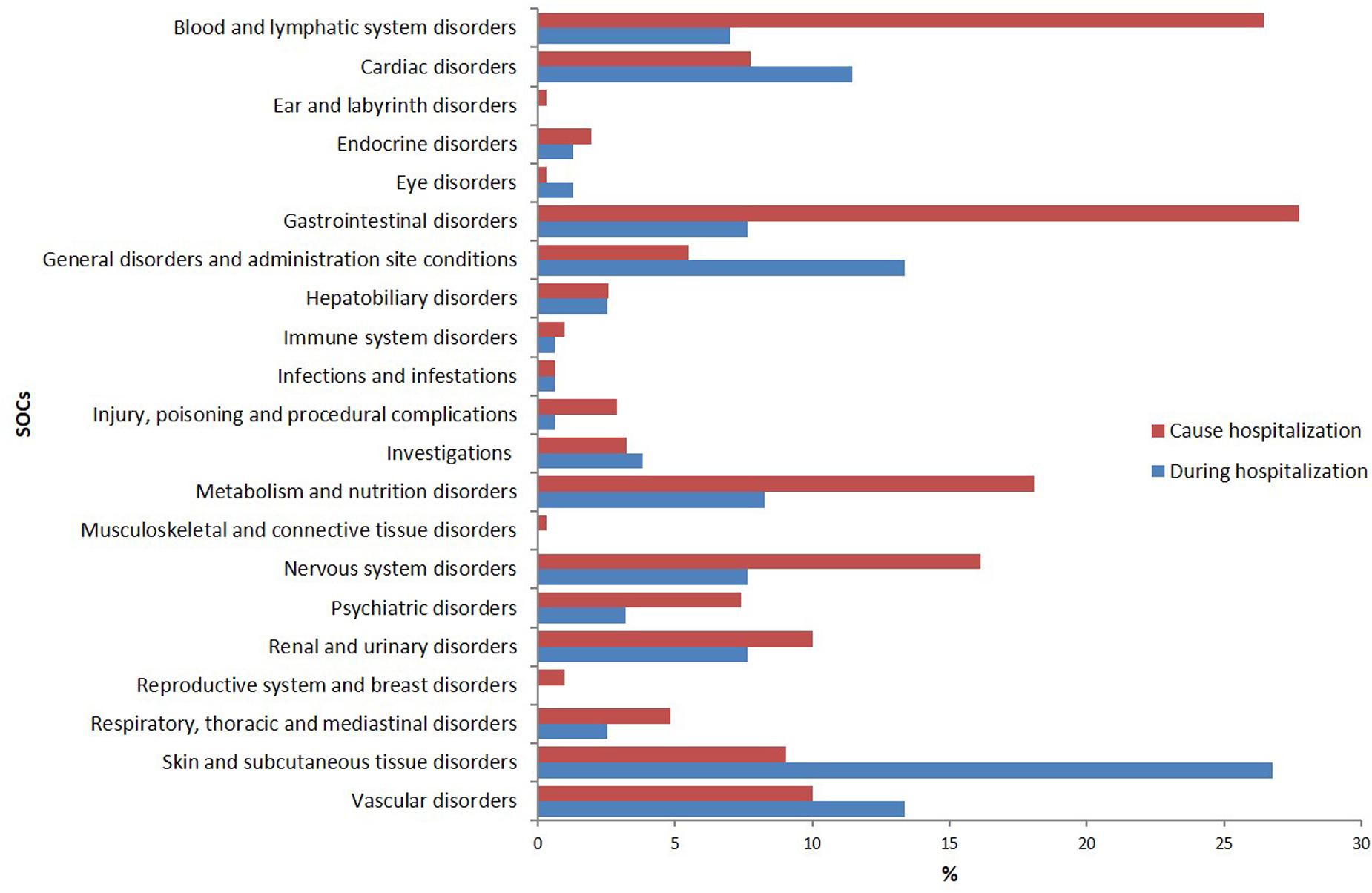
Frontiers | Adverse Drug Reactions in Hospitalized Patients: Results of the FORWARD (Facilitation of Reporting in Hospital Ward) Study | Pharmacology

Consequences to patients, clinicians, and manufacturers when very serious adverse drug reactions are identified (1997–2019): A qualitative analysis from the Southern Network on Adverse Reactions (SONAR) - eClinicalMedicine

The value of patient reporting to the pharmacovigilance system: a systematic review - Inácio - 2017 - British Journal of Clinical Pharmacology - Wiley Online Library

Consumer feedback on medicines vs Spontaneous ADR reporting | by Navro Technology Solutions | Medium
A Retrospective Analysis of Spontaneous Adverse Drug Reactions Reports Relating to Paediatric Patients | PLOS ONE
Analysis of the reporting of adverse drug reactions in children and adolescents in Germany in the time period from 2000 to 2019 | PLOS ONE

Reported adverse drug reactions in women and men: Aggregated evidence from globally collected individual case reports during half a century - eClinicalMedicine